Story at a glance…
-
When receiving a sample of a young mouse’s gut microbiome, age-related cognitive decline was reversed in older mice.
-
This included measures of anxiety, learning, and memory, but also immune functions in the brain.
-
While more research is needed, the finding joins a generous body of evidence suggesting fecal microbiome transplants could be key to ensuring a healthy aging process in humans.
Organ transplants are an extremely important medical procedure, but growing literature could see regular “fecal microbe transplants” given to us as we age, as animal studies showing the effect this bizarre intervention has on attenuating the aging process, particularly in the brain and gut, are promising.
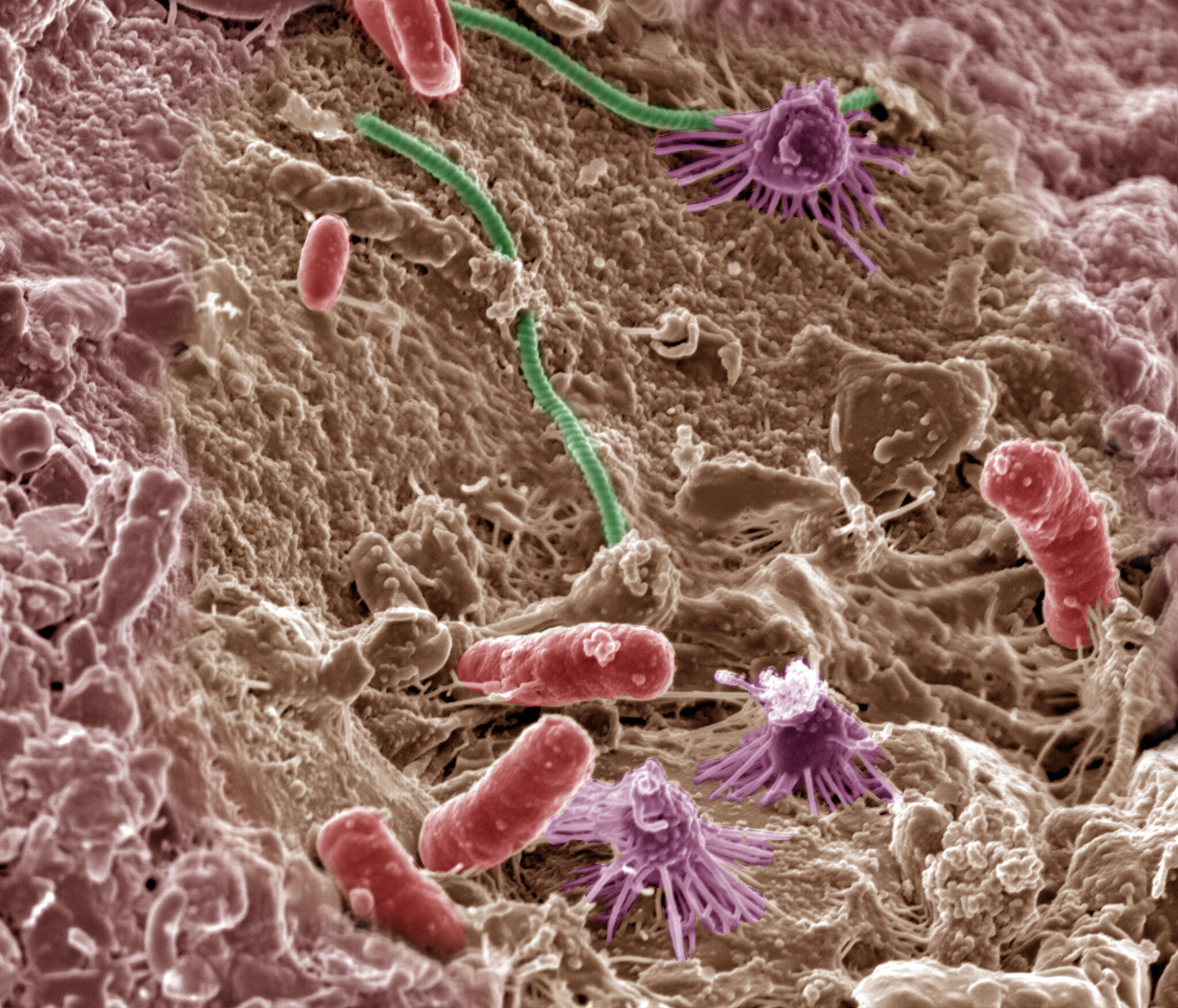
Now a new study published in Nature Aging, has found that transplanting a piece of the gut microbiome—the trillions of individual bacteria, viruses, and protozoa that live in our gastrointestinal system, from a younger mouse into an older one, reversed age-related changes in the immune function of the brain as well as returning the patterns of anxiety, learning, and memory, to those of a younger mouse.
Their study reported the fecal microbiome transplant (FMT) from a 3-4 month-old mouse into a 19-20 month-old mouse “reversed aging-associated differences in peripheral and brain immunity, as well as the hippocampal metabolome and transcriptome of aging recipient mice” as well as “attenuated selective age-associated impairments in cognitive behavior”.
The brains of old mice receiving young donor-derived fecal transplants were found after to contain metabolites and patterns of gene regulation that resembled the brains of the younger mice.
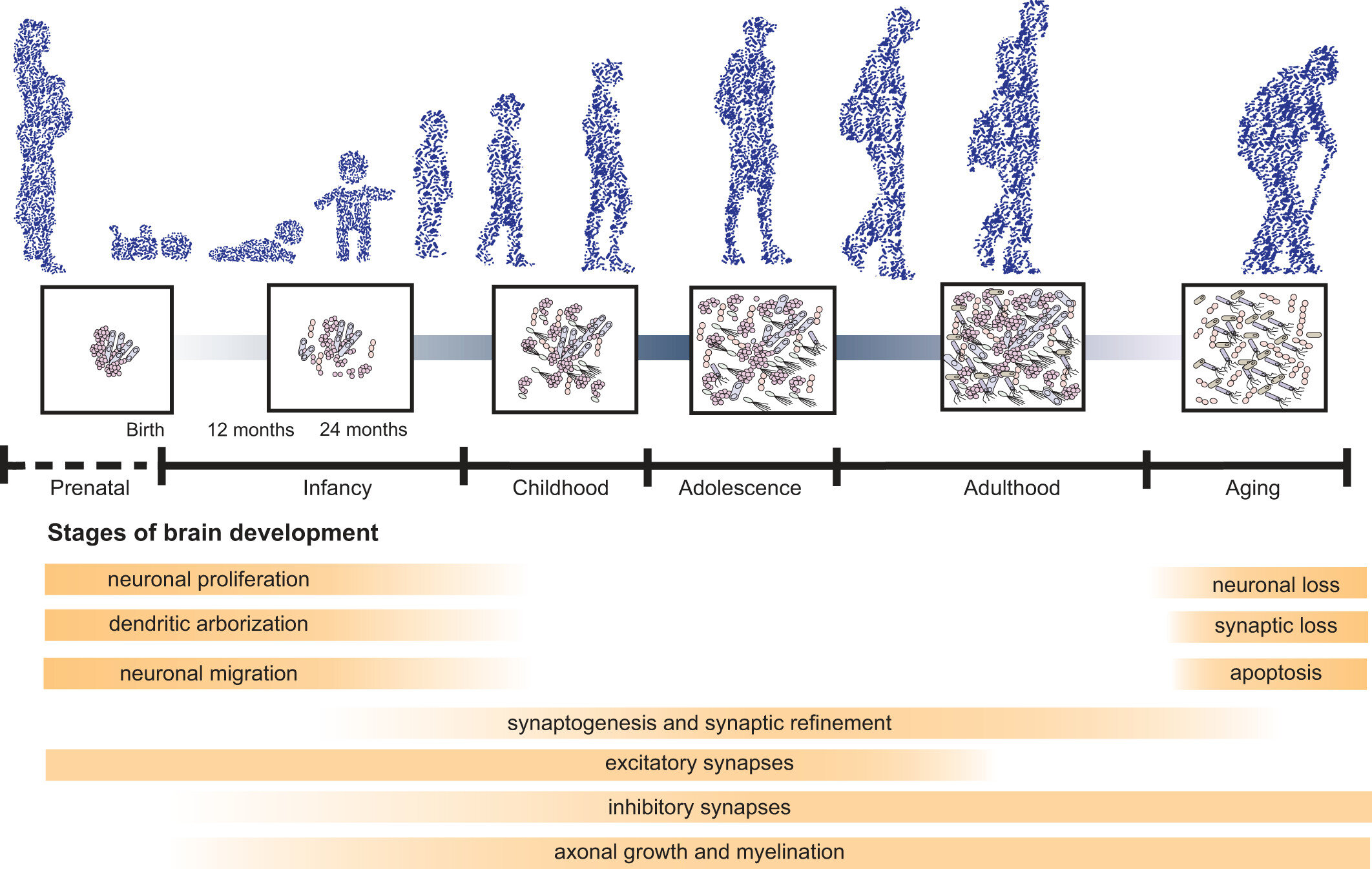
Aging is a complex process of systematic decline, and age-related changes in gut microbiota are linked with a decline in cognitive abilities in young rats receiving an FMT from old rats. This same study also found the aged FMT increased expression of pro-inflammatory cytokines and oxidative stress, suggesting the mechanism for gut-related cognitive decline during aging is driven by inflammation.
The age-related decline in microbiome diversity and richness is also linked to a poorer diet which in turn worsens the condition of the microbiome, worsening cognitive decline. One study found that separating 178 elderly patients by microbiome richness simultaneously separated them by diversity of diet, as well as by measures of frailty.